Time left =
2
The state of an ideal gas is changed in a closed path 1→2→3→4→1. Which of the following is true about work done on the gas? Work 1→2 Work 2→3 Work 3→4 Work 4→1
3
An ideal gas is taken from state 1 to state 2 and then to state 3. If the process 1-2 is adiabatic and 2-3 is isothermal, what is a true statement about the change in temperature and heat transferred during 1-2?
4
The state of an ideal gas is changed isothermally from position 1 to position 2 is shown above. What is the change in the internal energy of the gas during this process?
5
When we touch a piece of metal and a piece of wood that are placed in the same room, the piece of metal feels much colder than the piece of wood. This happens because of the difference in:
6
A hot object with a temperature T1 is connected to a cool object with a temperature of T2. The object used to conduct heat has a length L and a cross-sectional area A. The rate of heat flow is:
7
The process of heat transfer from object to another because of molecular motion and interaction is called:
8
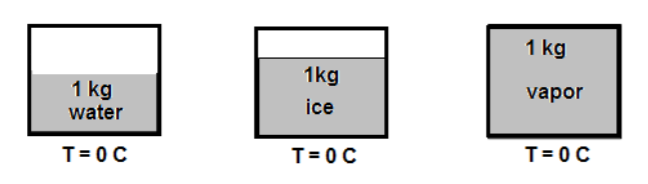
. Three containers filled with 1 kg of each: water, ice, and water vapor at the same temperature T = 0 °C. Which of the following is true about the internal energy of the substances?
9
. Mechanical equivalent is associated with:
10
A sample of ideal gas has an internal energy U and is then compressed to one-half of its original volume while the temperature stays the same. What is the new internal energy of the ideal gas in terms of U?
11
The absolute temperature of an ideal diatomic gas is quadrupled. What happens to the average speed of molecules?
12
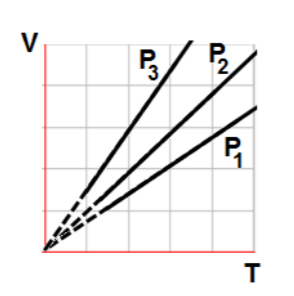
The state of an ideal gas was changed three times in a way that the volume stays the same. The graph represents three isobaric lines. Which of the following is true about the volume of the gas?
13
The temperature of an ideal gas increases from 20 °C to 40 °C while the pressure stays the same. What happens to the volume of the gas?
14
The state of an ideal gas was changed three times in a way that the pressure stays the same. The graph represents three isobaric lines. Which of the following is true about the pressure of the gas?
15
Which of two temperature change are equivalent?
16
Which of the following temperature scales doesn’t have negative numbers?
17
Three containers filled with 1 kg of each: water, ice, and water vapor at the same temperature T = 0 °C. Which of the following is true about the internal energy of the substances?
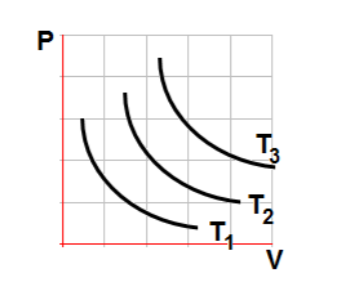
18
The state of an ideal gas was changed three times at three different temperatures. The diagram represents three different isothermal curves. Which of the following is true about the temperature of the gas?